
Although studies in structure–property-activity relationships go back to antiquity since the times of Crum-Brown and Fraser, it is only in the recent times that one witnesses a vigorous study of it as an interdisciplinary area of molecular design and modelling by developing quantitative relationship between molecular activity or property (such as partition coefficient (log P), boiling point, melting point, acid and base constant, chromatographic retention index, toxicity, or reactivity) and theoretical structural properties such as constitutional, electrostatic, geometrical, topological, or quantum chemical molecular characteristics. The concept of quantitative structure–property-activity relationship (QSAR/ QSPR) was originated from the idea of Crum-Brown and Fraser as early as 1870 when they proposed that biological response was a function of chemical structure. Structure-reactivity relationship is a variant of structure–property-activity relationship studies.
Homo lumo diels alder free#
The free energy difference between initial geometry-optimized reactants and the transition state at maximal potential energy barrier is correlated with the rate constant or reactivity of the reactant molecules. Still locating the transition state is not easy and therefore one has to proceed with computationally less demanding methods. For basically all the condensed phase reactions the transition state is valid and in order to calculated the rate constant it is enough to calculate the activation free energy. The free energy of activation is reversible work required to bring the system from the minimum of reactive well to the top of the barrier. It corresponds to the potential barrier separating the minima of potential energy of reactants and products.
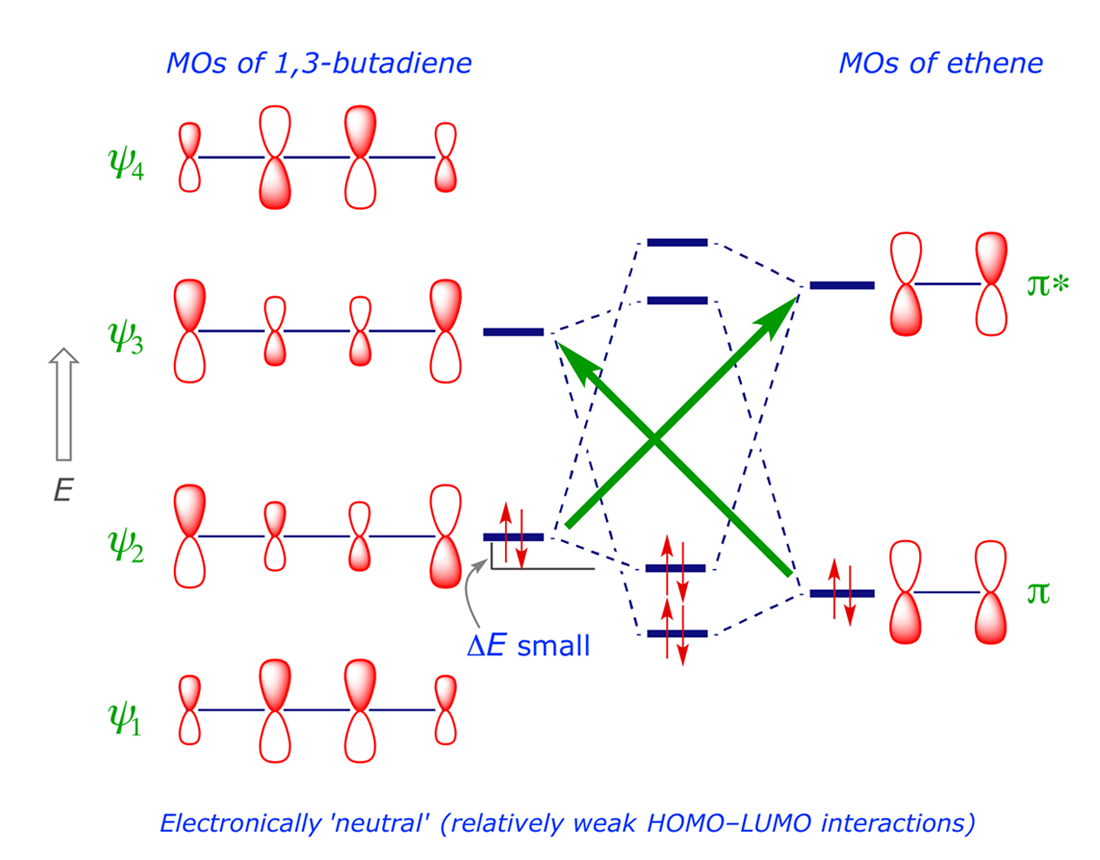
Thus, studies in the direction of QSABR modelling that provide efficient and fast prediction of activation barriers of the Diels-Alder reactions turn out to be a meaningful alternative to transition state theory based computation.Īctivation energy is the minimal energy required to start a chemical reaction. ConclusionsĪ reasonable predictability for the activation barrier of the test set reactions was obtained, which enabled an exploration and interpretation of the significant variables responsible for Diels-Alder interaction between dienes and dienophiles. Alternatively, a neural network model based on back propagation of errors was developed to assess the nonlinearity of the sought correlations between theoretical descriptors and experimental reaction barriers. The QSABR model can explain and predict 86.5% and 80% of the variances, respectively, in the activation energy barrier training data. Predictive performance of the quantitative structure-activation barrier relationship (QSABR) model was assessed by training and test set concept and by calculating leave-one-out cross-validated Q 2 and predictive R 2 values. Variable selection and model development were carried out by stepwise multiple linear regression methodology. Descriptors were computed using Hartree-Fock theory using 6-31G(d) basis set as implemented in Gaussian 09 software.
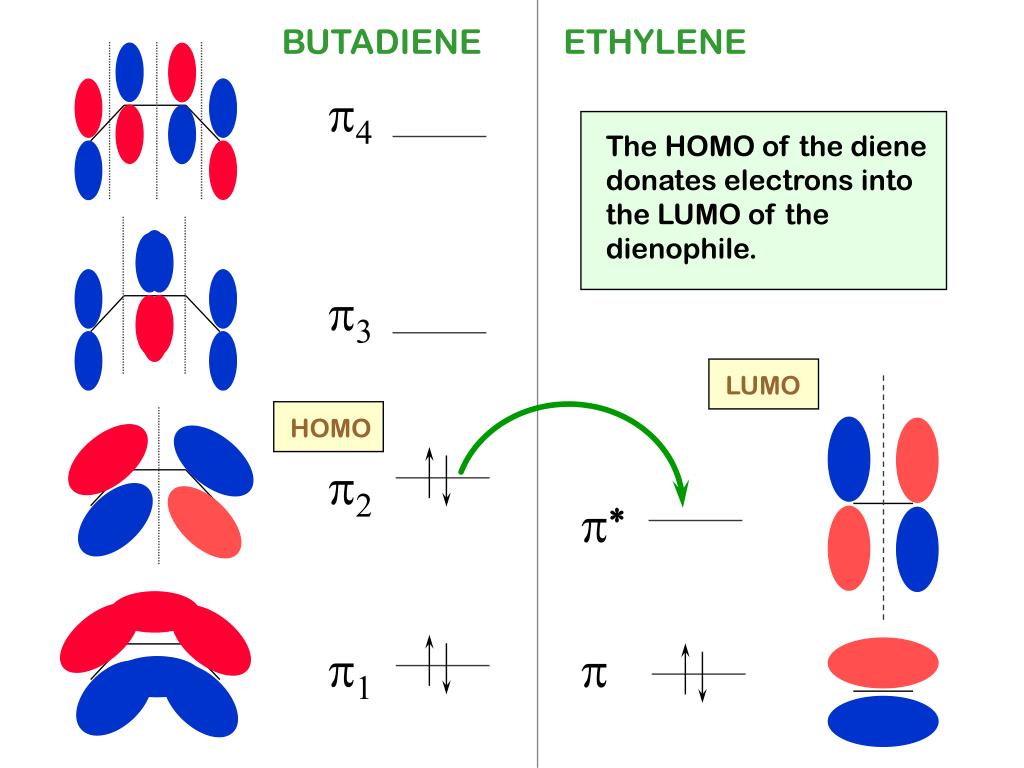
Experimental activation barrier data were obtained from literature.

A set of 72 non-catalysed Diels-Alder reactions were subjected to quantitative structure-activation barrier relationship (QSABR) under the framework of theoretical quantum chemical descriptors calculated solely from the structures of diene and dienophile reactants. In the present study, we show the correlation of quantum chemical structural descriptors with the activation barriers of the Diels-Alder ligations.


 0 kommentar(er)
0 kommentar(er)
